
Fluorogenic Chemogenetic Biosensors for Biological Imaging
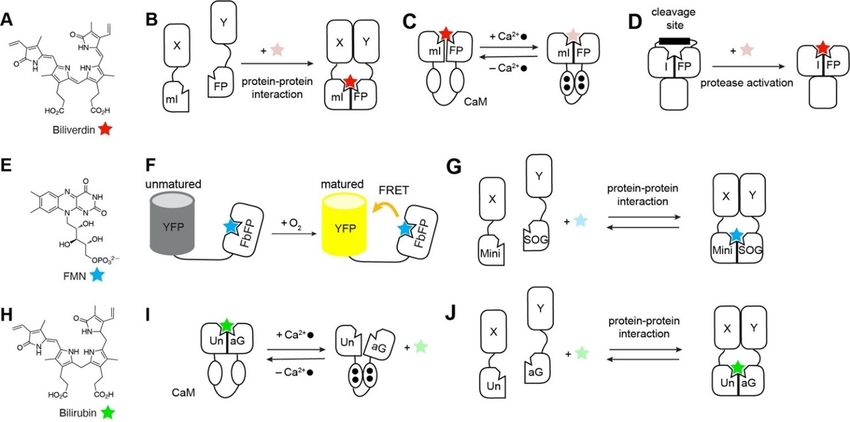
Fluorescent chemogenetic biosensors based on natural fluorogens. (A) Chemical structure of the endogenous fluorogen biliverdin (BV). (B) iSplit: irreversible reconstitution of mIFP upon protein‐protein interaction allows covalent BV binding, thus fluorescence activation. (C) NIR‐GECO1: Conformational change of the calmodulin sensing module upon calcium binding yields fluorescence inactivation of the complexed BV. (D) iProtease: release of the biliverdin binding site after protease cleavage allows covalent binding of BV, thus fluorescence activation. (E) Chemical structure of the endogenous fluorogen flavin mono‐nucleotide FMN. (F) FluBO: YFP chromophore maturation, thus FRET efficiency, depends on molecular oxygen levels. (G) Split miniSOG: reversible reconstitution assembly upon protein‐protein interactions allows non‐covalent binding of FMN, and thus fluorescence activation (H) Chemical structure of the endogenous fluorogen bilirubin (BR). (I) BReleaCa: conformational change of calmodulin upon Ca²⁺ binding reduces UnaG affinity for BR, thus fluorescence intensity. (J) uPPI: reversible reconstitution of UnaG upon protein‐protein interaction allows non‐covalent binding of BR, thus fluorescence activation.
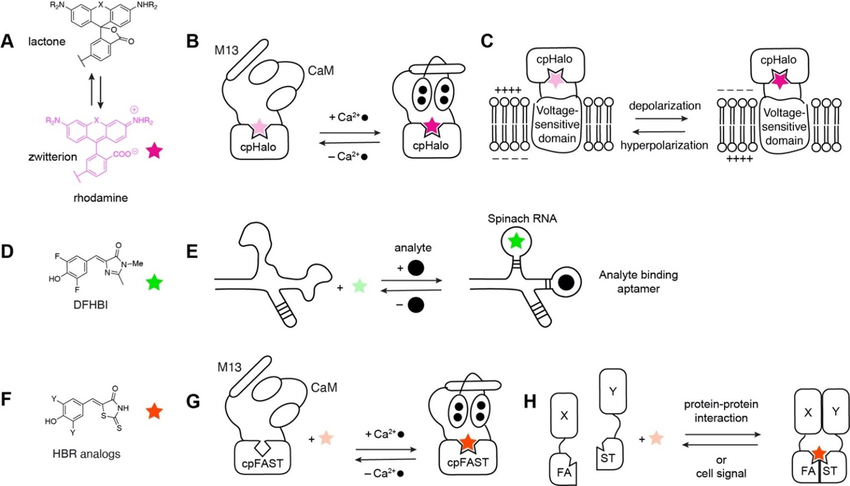
Fluorescent chemogenetic biosensors based on synthetic fluorogens. (A) Chemical structure of synthetic fluorogenic rhodamines. (B) HaloCaMP: upon Ca²⁺ binding, conformational change of the sensing module CaM/M13 yields fluorescence activation of the rhodamine dye covalently linked to a circularly permuted (cp) HaloTag. (C) HArcLight: conformational change of a voltage‐sensitive domain yields fluorescence activation of the rhodamine dye covalently linked to a circularly permuted HaloTag. (D) Chemical structure of the synthetic fluorogen 3,5‐difluoro‐4‐hydroxybenzylidene imidazolinone (DFHBI) (E) RNA aptamers: Conformational change of the sensing module upon analyte complexation allows non‐covalent binding of DFHBI, thus fluorescence activation. (F) Chemical structure of the synthetic fluorogenic HBR analogs. (G) FAST‐based Ca²⁺ sensor: conformational change of the sensing module CaM/M13 increases affinity for the HBR analog, thus fluorescence intensity. (H) split FAST: reversible reconstitution of FAST upon assembly of the sensing module allows non‐covalent binding of the HBR analog, thus fluorescence activation.
'Review' 카테고리의 다른 글
| plz read (0) | 2024.10.30 |
|---|---|
| Rapamycin passes the torch: a new generation of mTOR inhibitors (0) | 2024.10.28 |
| homo lumo (0) | 2024.10.28 |