
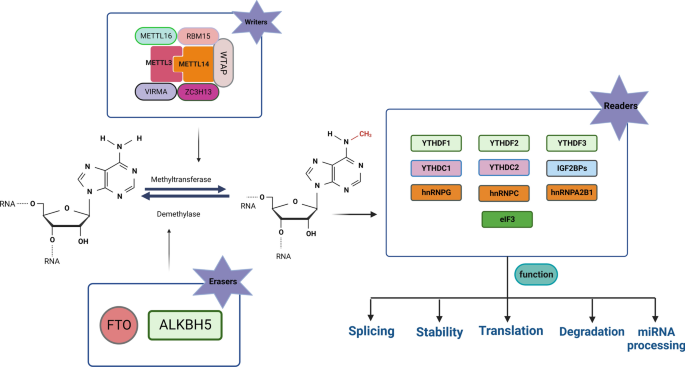
1. Writers
Writers are enzymes that add chemical modifications to RNA molecules. They establish the modification marks on RNA, such as methylation or acetylation.
Examples of Writers:
m6A Methyltransferases: Add m6A modifications.
METTL3-METTL14 complex: Core m6A methyltransferase.
WTAP: A cofactor that enhances m6A writing.
m5C Writers:
NSUN family proteins: Add m5C to tRNA, rRNA, and mRNA.
Pseudouridine Synthases:
PUS enzymes: Convert uridine to pseudouridine.
2. Readers
Readers are proteins that recognize and bind to specific RNA modifications, interpreting them to influence RNA function, stability, translation, or localization.
Examples of Readers:
YTH Domain Proteins: Recognize m6A modifications.
YTHDF1: Enhances translation of m6A-modified mRNA.
YTHDF2: Promotes degradation of m6A-modified mRNA.
ALYREF: Binds to m5C-modified mRNA and facilitates its export from the nucleus.
EIF3: Recognizes m6A in the 5′ UTR to initiate translation.
3. Erasers
Erasers are enzymes that remove RNA modifications, making the process reversible and allowing dynamic regulation of RNA functions.
Examples of Erasers:
m6A Demethylases:
FTO (Fat Mass and Obesity-Associated Protein): Removes m6A or m6Am marks.
ALKBH5: Specifically demethylates m6A.
TET Enzymes: Convert m5C into hydroxymethylcytosine (hm5C) or facilitate its removal.
Dynamic Regulation
The interplay of writers, readers, and erasers creates a dynamic and flexible system, allowing cells to respond to environmental signals and regulate gene expression. For example:
Writers add an m6A mark on mRNA to regulate stability.
Readers bind to the m6A-modified RNA to influence translation or degradation.
Erasers remove the m6A mark when the regulation is no longer needed.
This coordinated system ensures precise control of RNA metabolism and cellular processes, including development, stress response, and disease progression.
1. Methylation
m6A (N6-Methyladenosine): Methylation at the N6 position of adenine, the most abundant RNA modification in eukaryotic mRNAs.
m1A (N1-Methyladenosine): Methylation at the N1 position of adenine, found in tRNAs and rRNAs.
m5C (5-Methylcytosine): Methylation at the 5th carbon of cytosine, present in tRNAs, rRNAs, and mRNAs.
m7G (7-Methylguanosine): Found at the 5' cap of eukaryotic mRNAs and in some tRNAs.
2′-O-Methylation: Methylation at the ribose 2′-hydroxyl group, common in rRNA and snRNA.
2. Acetylation
N4-Acetylcytidine (ac4C): Acetylation at the N4 position of cytidine, found in tRNA and mRNA, enhancing mRNA stability and translation.
3. Pseudouridylation
Pseudouridine (Ψ): Isomerization of uridine, where the uracil base is attached to the sugar via a C-C bond instead of a N-C bond. Found in tRNA, rRNA, and snRNA, contributing to structural stability.
4. Inosine Modification
Inosine (I): Formed by the deamination of adenosine, commonly found in tRNA and mRNA, where it plays a role in wobble base pairing and RNA editing.
5. Thiolations
s2U (2-Thiouridine): Sulfur replaces oxygen at the 2′ position of uridine, found in tRNA, enhancing its stability and function.
6. Isopentenylation
i6A (N6-Isopentenyladenosine): Addition of an isopentenyl group to adenine in tRNA, important for codon-anticodon recognition.
7. Hydroxylation
hm5C (5-Hydroxymethylcytosine): Hydroxymethylation of cytosine, found in RNA of certain organisms, with emerging roles in gene expression.
8. Queuosine Modification
Q (Queuosine): A complex modification of guanosine in tRNA, influencing translation fidelity.
9. U-to-Ψ Editing
Conversion of uridine to pseudouridine in RNA molecules.
10. ADAR-mediated Editing
A-to-I RNA Editing: Adenosine is converted to inosine by adenosine deaminases acting on RNA (ADARs), affecting coding potential and RNA stability.
These modifications collectively regulate RNA structure, function, and interaction with proteins, making them crucial for cellular function and adaptation.
'Trends•Issues•Papers' 카테고리의 다른 글
| 30th organic synthesis seminar (0) | 2025.03.24 |
|---|---|
| chemoinforgraphic (0) | 2025.01.23 |
| Long-term injectable drug delivery systems (DDS) (0) | 2024.11.26 |
| open innovation conference (0) | 2024.11.23 |
| effective talk tip (0) | 2024.11.02 |