There are a wide variety of experiments that can be performed using flow cytometry.
1) Experiment using fluorescent antibodies
As we have already covered to some extent when introducing flow cytometry, the main purpose of flow cytometry is to find out and analyze what kind of cell it is by analyzing various proteins expressed by the cell. Therefore, many fluorescent antibodies that can specifically bind to various proteins (antigens) have already been developed, and these fluorescent antibodies can be used to analyze what proteins the cells are expressing. These various protein expression profiles are a way to distinguish the types of immune cells.

Although only membrane proteins present on the cell surface were used as examples above, transcriptional regulators (Fig. 4B) and cytokines (Fig. 4C) present inside cells can also be measured by staining with fluorescent antibodies. In the case of cytokines, they are proteins secreted by cells, but they can be measured by accumulating cytokines inside cells (Golgi and ER) using protein transport inhibitors.

The use of fluorescent antibodies is not limited to simply checking the presence or absence of proteins. Interestingly, it is also possible to check the amount of phosphorylated proteins. For example, when CD4+ T cells are activated, MAPK signals are activated and ERK proteins are phosphorylated. Using fluorochrome-conjugated anti-phospho-ERK antibodies, the amount of phosphorylated ERK can be measured at the cellular level of CD4+ T cells (Fig. 5). Of course, since the types of fluorescent antibodies developed for flow cytometry are fewer than those developed for Western blot, there are still some limitations in analyzing the degree of phosphorylation by flow cytometry, but it has a great advantage in analyzing at the cellular level. Fluorescent antibodies for flow cytometry are being developed by companies such as eBioscience, BD Bioscience, and Biolegend, and although the principles are similar, not all fluorescent antibodies are compatible between flow cytometry and confocal microscopy. Some fluorescent antibodies may be usable in flow cytometry, but may not be suitable for fluorescence microscopy due to weak or unstable signals.
2) Fluorescent protein analysis
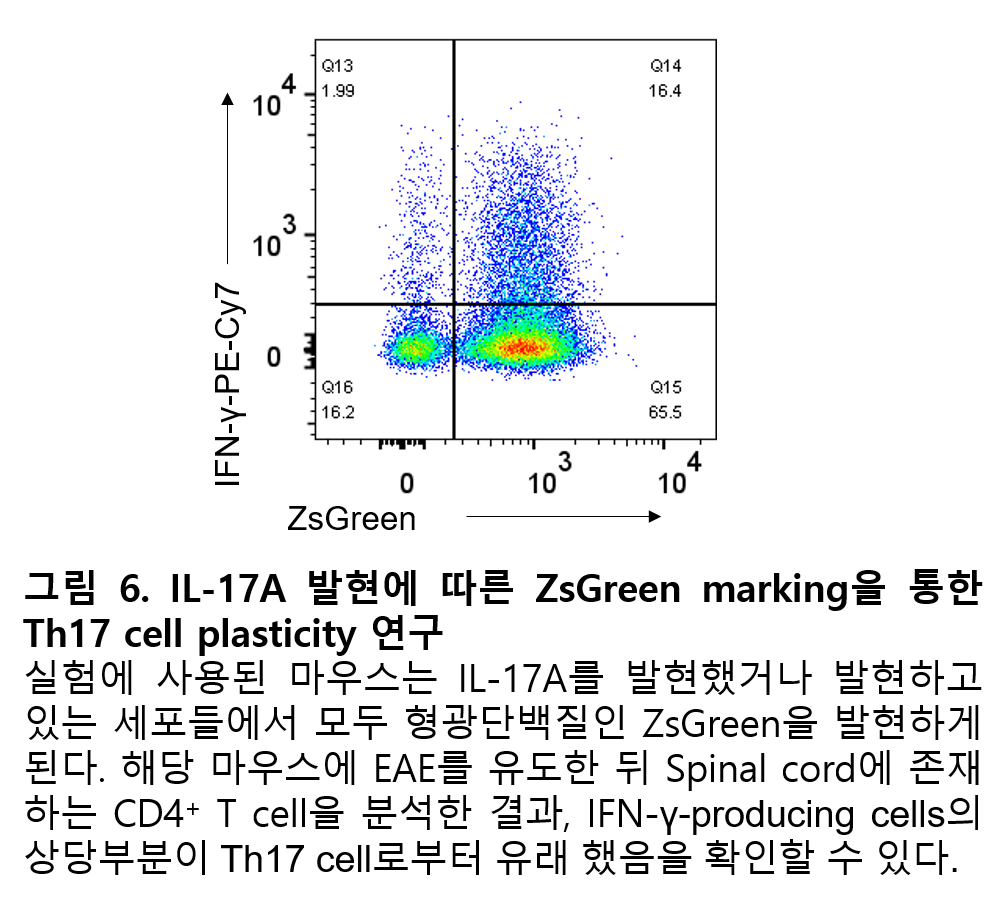
You may have heard a lot about fluorescent proteins such as GFP (Green fluorescence protein). There are many mice that have been genetically engineered to express such fluorescent proteins in their cells, and fluorescent proteins expressed in their cells can also be measured using flow cytometry. Using these fluorescent proteins and flow cytometry, it is possible to study cell plasticity by identifying what type of cells a specific immune cell was previously. For example, in the case of Il17aCre Rosa26YFP mice, YFP is continuously expressed in cells that have previously expressed a cytokine called IL-17A. It should be noted that once IL-17A is expressed even once, YFP is continuously expressed, so cells that have previously expressed IL-17A are labeled with YFP regardless of whether or not they currently express IL-17A. The development of these mice and the use of flow cytometry have contributed to expanding our understanding of Th17 cell plasticity by demonstrating that most CD4+ T cells expressing IFN-γ in EAE, an animal model of multiple sclerosis, are derived from Th17 cells that express (or did express) IL-17A (Hirota et al., 2011) (Fig. 6).
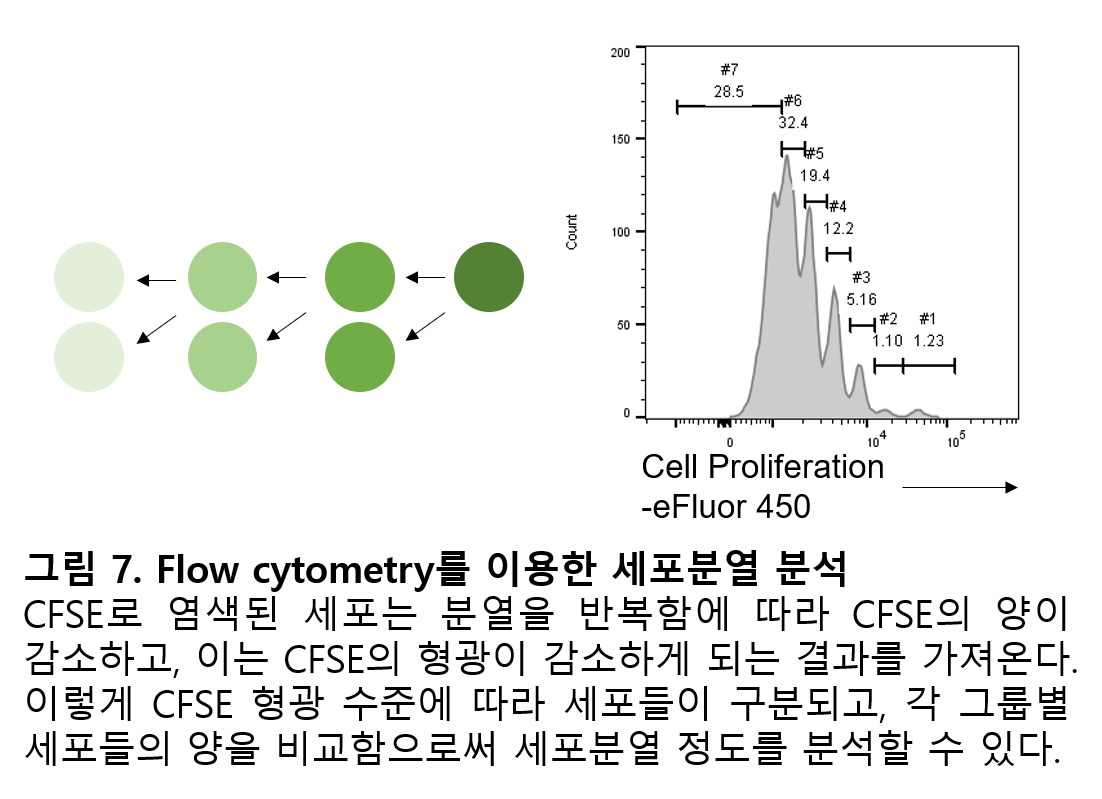
3) Experiment using fluorescent dye: Cell proliferation
The basis of flow cytometry is measuring fluorescence, but fluorescent substances are not necessarily used only by attaching to antibodies. One widely used, but not an antibody, is CFSE. CFSE is a fairly stable fluorescent substance that can penetrate the cell membrane and bind nonspecifically to proteins inside the cell (Parish, 1999). Therefore, when cells are stained with CFSE, all stained cells are evenly stained with CSFE, and CFSE continues to remain inside the cell. And each time a cell divides, the cytoplasm is divided into two, and the amount of CFSE held by each cell is reduced by half, which ultimately results in the fluorescence that a single cell can produce being reduced by half. In this way, as the cell divides n times, the fluorescence of CFSE theoretically decreases to 1/2n (Fig. 7). When cells stained with CFSE divide and are analyzed by flow cytometry, the cells are classified according to the level of CFSE fluorescence, and by comparing the cells in each group, the extent of cell division can be analyzed. Currently, various fluorescent dyes that have the same principle as CFSE but have different fluorescence are being utilized.
4) RNA Measurement: Application of FISH
The above are techniques for specifically or non-specifically staining proteins with fluorescent dyes and analyzing them using flow cytometry. Surprisingly, however, flow cytometry can also be used to measure RNA. This is a technique that applies FISH (Fluorescent in situ hybridization), and since I have not actually tried it yet, I will explain this part by referring to the Primeflow RNA Assay product developed by Invitrogen.
The core of this technology is to create a probe that can recognize the RNA you want to measure, bind it to RNA, and amplify the fluorescent signal that the probe can produce. If you label the probe that recognizes RNA with fluorescent dye, the intensity of the fluorescence that a single probe can produce is very weak, so a process of amplifying this signal is necessary. Therefore, by additionally adding DNA sequences (Amplifiers) that recognize the probe and attaching a fluorescent labeling probe later, the fluorescent signal is amplified (Figure 8). It is similar to the principle of amplifying the signal by attaching a secondary antibody to the primary antibody. Despite this simple principle, the product price is quite expensive, so it is a technology with a little low accessibility, but it is a groundbreaking experimental technique in that it can compare the level of protein and RNA expression at the cellular level.

5) Measuring changes in intracellular calcium concentration
We have previously introduced methods for measuring proteins and RNA using flow cytometry. However, the scope of application of flow cytometry extends beyond measuring proteins and RNA to measuring changes in the concentration of metal ions within cells. There are many examples, but measuring changes in intracellular calcium concentration is a representative example.
One of the most rapid phenomena that occurs when immune cells are activated is the increase in intracellular calcium ions. There are calcium indicators such as Fluo-4, Fluo-5, and Indo-1 that bind to this increased calcium ion and change their fluorescence characteristics, and these calcium indicators have the property of easily penetrating into cells. Therefore, if the calcium indicator is permeated into the cell and an appropriate response is given to the cell to induce changes in calcium concentration, the fluorescence caused by the calcium indicator bound to calcium can be measured in real time (Figure 9).

Not only that. Calmodulin and M13 domains, which are calcium-binding proteins, can be fused to fluorescent proteins to measure intracellular calcium changes without a separate staining process (GECI: Genetically encoded calcium indicators). The principle is that the structural changes in the protein caused by calcium binding to Calmodulin and M13 domains change the fluorescence properties of the fluorescent protein (Zhong and Schleifenbaum, 2019). Using mice that express the protein in the body, the activity of immune cells occurring in vivo can also be measured in real time.
Calcium was explained as an example, but there are various fluorescent probes that can detect changes in the concentration of metal ions such as sodium and zinc, changes in intracellular pH, and changes in ROS concentration, and these intracellular changes can be measured using flow cytometry.
6) FACS (Fluorescence-activated cell sorting)
The last technique to be introduced is FACS, which is the pinnacle of technology utilizing flow cytometry. It is a sorting technique that sorts only specific cells from cells analyzed by flow cytometry. Many people use the same meaning for flow cytometry and FACS, but in fact, they are different. FACS is a technique that sorts cells using flow cytometry. FACS is a technique that applies a positive or negative charge (electronic charge) to droplets containing desired cells through a device additionally installed in a general flow cytometry machine, and attracts the corresponding droplets with the charge to sort the corresponding cells.
Since the cells sorted in this way are living cells, additional culture is possible. In addition, only the cells obtained in this way are used for bulk RNA-seq or single cell RNA-seq analysis.
References
Hirota, K., Duarte, J.H., Veldhoen, M., Hornsby, E., Li, Y., Cua, D.J., Ahlfors, H., Wilhelm, C., Tolaini, M., Menzel, U., et al. (2011). Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12, 255-263.
Parish, C.R. (1999). Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol 77, 499-508.
Zhong, C., and Schleifenbaum, J. (2019). Genetically Encoded Calcium Indicators: A New Tool in Renal Hypertension Research. Front Med (Lausanne) 6, 128.
[BRIC Bio Correspondent] [Immunology Research Notes I Want to Give to My Juniors] #2_Flow Cytometry_Introduction
'Lab skills' 카테고리의 다른 글
| sc rna seq workflow (0) | 2025.01.01 |
|---|---|
| how flow cytometry contributes in achieving specific research goal (0) | 2024.10.26 |
| fluorescence Principle and Dyes (0) | 2024.10.26 |
| Rna FISH (0) | 2024.10.26 |
| research objective and method (0) | 2024.10.26 |